Early allograft dysfunction and primary nonfunction
Primary graft dysfunction is one of the most common early complications following liver transplantation. It is also considered one of the most feared by transplant surgeons due to its severe consequences, which may lead to the need for retransplantation or even death.
Primary graft dysfunction can be classified into:
- Early allograft dysfunction (EAD): initial, transient graft dysfunction with potential for recovery.
- Primary non-function (PNF): irreversible and severe form of dysfunction with no identifiable cause.
Currently, most authors consider both concepts as different degrees of the same complication, with PNF representing its most severe expression.
The incidence of primary graft dysfunction varies across different series, partly due to the lack of consensus in its definition. In the case of EAD, it ranges from 15% to 30%, while PNF has an incidence of between 2% and 9%.
Liver graft failure leads to a higher number of postoperative complications, increased mortality, and a greater risk of graft loss.
Causes
The risk factors associated with primary graft dysfunction have remained consistent over the years. These can be divided into three groups depending on whether they are related to the donor, the surgical procedure itself, or the recipient.
Donor-related risk factors that have traditionally been associated with a higher risk of graft dysfunction include donor age, female sex, body mass index (BMI), length of stay in the intensive care unit (ICU), degree of steatosis, and donor type (e.g. DCD donors).
Currently, the number of patients on the liver transplant waiting list exceeds the number of available donors. This imbalance has driven the development of strategies aimed at increasing organ availability, particularly through broader donor selection.
A first step was the introduction of donation after circulatory death (DCD), which led to an increase in organ availability. However, in the early stages, organs from DCD donors showed a higher incidence of early allograft dysfunction (EAD), largely due to ischaemia–reperfusion injury (IRI) and prolonged warm ischaemia times associated with the rapid recovery technique used during donation, with rates of EAD reaching up to 68.4%.
These outcomes have improved with the advent of in situ perfusion technologies (such as ECMO) and ex situ machine perfusion, both of which have significantly impacted the increase in donor numbers. In fact, the use of ECMO in Spain has overtaken the formerly preferred super-rapid retrieval technique, reducing the incidence of EAD and ischaemic cholangiopathy associated with DCD donors.
ECMO has also enabled multi-organ donation in DCD, which accounted for 45% of all donors in Spain in 2023.
Despite the introduction of DCD, organ shortage remained a pressing issue. This led to further expansion in donor selection with the use of "expanded criteria donors", particularly in recipients with urgent need for transplantation.
Although there is no universally accepted definition for this concept, the main characteristics cited are shown in Figure 2.
All of this has led to the use of marginal donors, who carry a higher risk of EAD and, of course, of primary non-function (PNF).
The relationship between donor characteristics and graft dysfunction is so evident that the “Donor Risk Index” proposed by Feng was introduced as a tool to predict graft dysfunction. This index is based on a scoring system that incorporates various donor features associated with increased risk of EAD and can be used to support improved allocation of available grafts.
Detailed information about surgical related causes would go here. This may involve aspects like surgical technique, duration of surgery, and intraoperative events.
Perioperative factors that have been associated with this complication include the duration of the transplant procedure itself, cold ischaemia time, warm ischaemia time, and the transfusion of blood products.
Recipient-related factors associated with graft dysfunction include the preoperative Model for End-stage Liver Disease (MELD) score, age, race, body mass index (BMI), and the presence of pre-transplant renal dysfunction.
Ischaemia-reperfusion injury (IRI)
EAD and PNF have been linked to ischaemia-reperfusion injury (IRI), and are considered a manifestation of this process. IRI refers to a condition in which cellular damage in a hypoxic organ is exacerbated following the restoration of oxygen. Some authors suggest that IRI may also be influenced by specific characteristics of both the donor and the recipient.
IRI consists of two distinct phases:
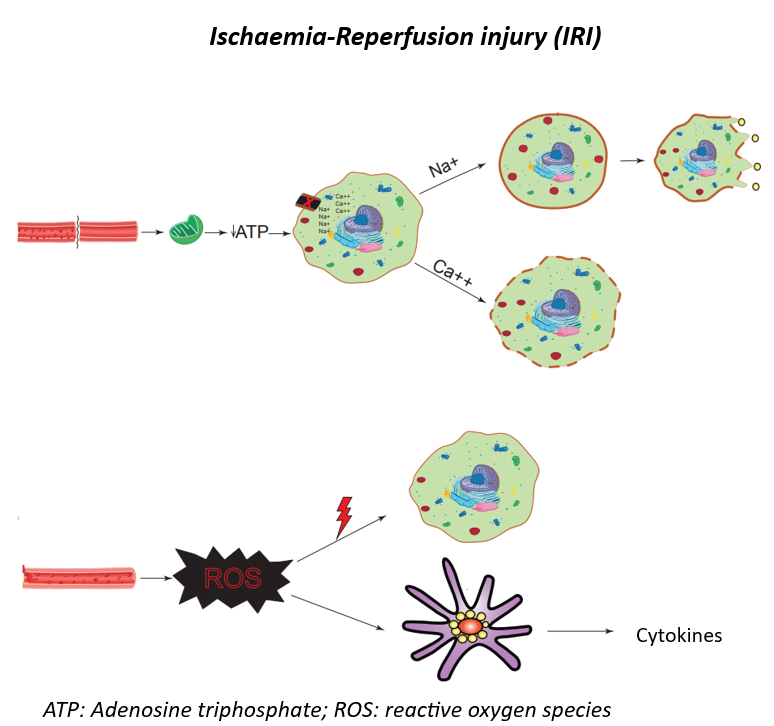
ATP: Adenosine triphosphate; ROS: reactive oxygen species
In the first phase, oxygen deprivation leads to mitochondrial dysfunction, resulting in adenosine triphosphate (ATP) depletion and subsequent failure of ATP-dependent membrane pumps. This disruption of the membrane impairs the homeostasis of H+, Na+, and Ca2+, leading to activation of hydrolytic enzymes and loss of cell volume regulation. Sodium influx causes oedema and cell death, while calcium influx activates phospholipases that lead tomembrane rupture. Moreover, intracellular calcium serves as a substrate for the generation of reactive oxygen species (ROS), which are characteristic of the second phase.
After graft reperfusion, the second and most damaging phase occurs. The reintroduction of oxygen to the ischaemic tissue leads to the release of ROS, which directly damage cells and stimulate Kupffer cells to secrete pro- inflammatory cytokines such as tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). In addition, neutrophil infiltration into the graft further amplifies the damage through ROS production and protease release.
Altogether, this cascade creates the underlying substrate for the development of EAD or PNF, increasing the risk of graft loss in both the short and long term. Furthermore, it has been associated with acute kidney injury in the postoperative period of liver transplantation and may even progress to multi-organ failure.
The main target cells of IRI are hepatocytes and sinusoidal endothelial cells (SECs). However, each cell type is affected by a different form of ischaemia.
- Hepatocytes: affected by warm IRI due to cytokine release mediated by Kupffer cells.
- SECs: vulnerable to cold IRI, which leads to narrowing of the vascular lumen and microcirculatory dysfunction.
